RS09/Toll Like Receptor (TLR) 4 agonist – APPHALS – CAS 1449566-36-2
Toll Like Receptors (TLRs)
Toll Like Receptors (TLRs) are an important family of type I transmembrane Pattern Recognition Receptors (PRRs) that play a key role in activating and linking the innate immune system to adaptive immunity,by sensing invading pathogens or endogenous damage signals. TLRs are expressed on sentinel cells,including macrophages and dendritic cells,and there are ten functional receptors in human (TLR1–10) and twelve in mice (TLR1−9,11−13).
TLR4 and LPS recognition
When TLRs are activated,they recruit specific adaptor proteins into the cytosol of immune cells. These adaptors act as messengers to activate transcription factors NF-κB and IRFs,which are the two major effectors of the Innate Immune Response (IIR).
Among membrane-based TLRs,TLR4 is well known for recognizing lipopolysaccharides (LPS) on myeloid cells surface. These cells express LY96 (or MD-2) and CD14 proteins at high level,which are used in collaboration with TLR4 to mediate signal transduction.
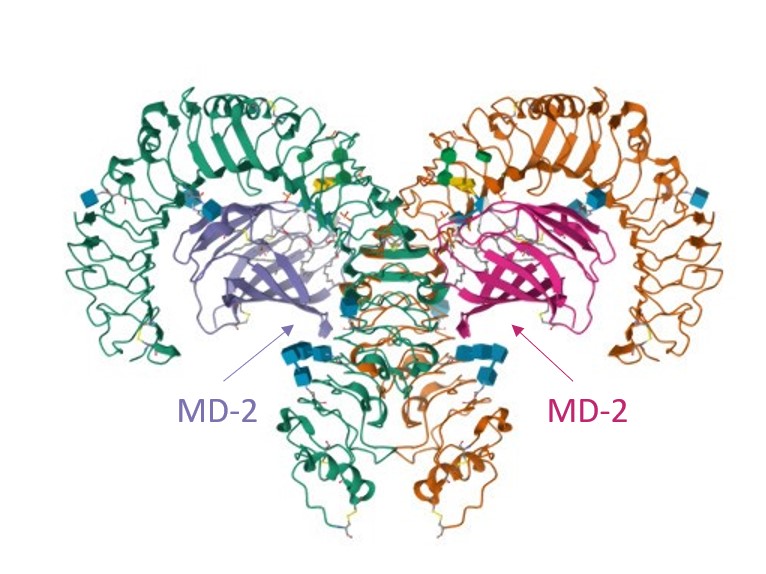
Recruitment of intracellular adaptor proteins requires dimerization of the TLR4/MD-2 complex,initiated by LPS binding to Lipopolysaccharide Binding Proteins (LBPs). This LPS-LBP complex will then be recognized by CD14 that will transfer LPS to MD-2,which is associated with TLR4 on the cell surface and without which LPS recognition by TLR4 would fail.
RS09 agonist – APPHALS peptide
APPHALS peptide is an LPS agonist used as vaccine’s adjuvant to increase antibody production in-vivo. Nowadays,most of the vaccines are formulated with aluminum-based adjuvants that lead to various side effects. The aim of adjuvants being to catalyze innate immune response by stimulating the interactions between antigens and TLR proteins,new TLR agonists based on bacterial material appeared as natural candidate.
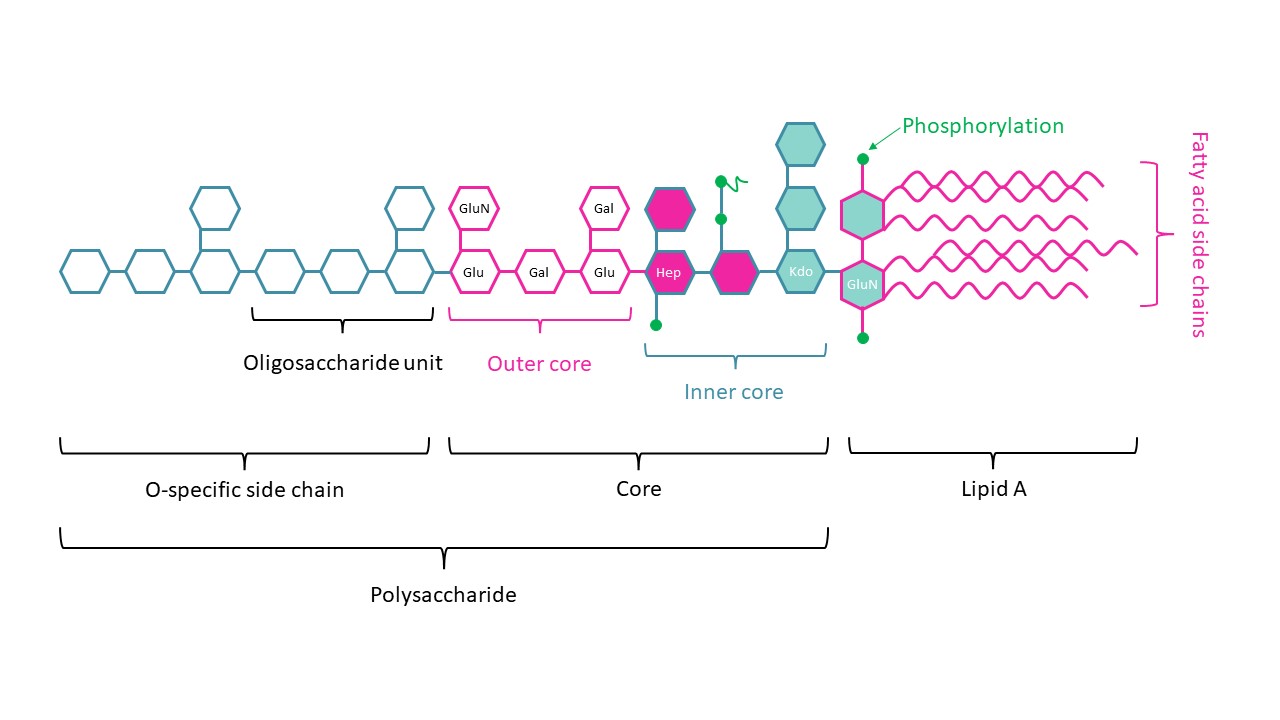
LPS being a key component of gram negative bacteria cell walls triggering TLR4 activation,the use of synthetic LPS peptides to mimic LPS-TLR4 interaction has emerged as a promising alternative adjuvant.
Technical specifications
 |
Sequence : APPHALS |
 |
MW : 691 ,78 g/mol (C31H49N9O9) |
 |
Purity : > 95% |
 |
Counter-Ion : TFA Salts (see option TFA removal) |
 |
Delivery format : Freeze dried in propylene 2mL microtubes |
 |
Peptide Solubility Guideline |
 |
Bulk peptide quantities available |
Price
| Product catalog | Size | Price € HT | Price $ USD |
| SB305-1MG | 1 mg | 77 | 96 |
| SB305-5MG | 5 mg | 270 | 337 |
| SB305-10*5MG | 50 mg | 1078 | 1348 |
References
Bull Natl Res Cent. 2019 Dec 12;43:187. doi: https://doi.org/10.1186/s42269-019-0227-2
Toll-like receptors activation,signaling,and targeting: an overview
Background
Toll-like receptors (TLRs) are an important family of receptors that constitute the first line of defense system against microbes. They can recognize both invading pathogens and endogenous danger molecules released from dying cells and damaged tissues and play a key role in linking innate and adaptive immunity. TLRs are widely distributed in both immune and other body cells. The expressions and locations of TLRs are regulated in response to specific molecules derived from pathogens or damaged host cells. The binding of ligands to TLR activates specific intracellular signaling cascades that initiate host defense reactions. Such binding is ligand-dependent and cell type-dependent and leads to production of pro-inflammatory cytokines and type 1 interferon. TLR-dependent signaling pathways are tightly increased during innate immune responses by a variety of negative regulators. Overactivation of TLRs can ultimately lead to disruption of immune homeostasis and thus increase the risk for inflammatory diseases and autoimmune disorders. Antagonists/inhibitors targeting the TLR signaling pathways have emerged as novel therapeutics to treat these diseases.
Aim of work
The present review summarizes the structure,characterizations,and signaling of TLRs and their regulators,as well as describes the implication of TLRs in many diseases with a brief idea about the inhibitors that target TLR signaling pathways.
Conclusion
We conclude that TLRs are the main elements of our immune system,and they should be maintained functioning to keep the integrity of innate immunity. Targeting of TLR signaling represents a new challenge for treatment of many diseases.
Mol. Pharmaceutics. 2016 Jan 29;13(3):885–894. doi: https://doi.org/10.1021/acs.molpharmaceut.5b00802
Intranasal Vaccination against HIV-1 with Adenoviral Vector-Based Nanocomplex Using Synthetic TLR-4 Agonist Peptide as Adjuvant
Recombinant type 5 adenovirus (rAd5) vaccines hold the promise to prevent HIV-1 infections. Intranasal vaccination not only stimulates systemic immunity but also elicits mucosal immunity that provides first defense for mucosally transmitted diseases like HIV-1. Adjuvants such as TLR agonists are usually codelivered with antigens to enhance the immunogenicity of vaccines. Here,we present a rAd5 vaccine delivery system using DEG-PEI as the carrier. Adenovirus encoding HIV gag was used as antigen,and was complexed with DEG-PEI polymer via electrostatic interaction. A novel synthetic TLR-4 agonist,RS09,was either chemically linked with DEG-PEI (DP-RS09) or physically mixed with it(DP/RS09) to enhance the immunogenticity of rAd5 vaccine. After intranasal immunization,the systemic antigen-specific immune responses and cytotoxicity T lymphocytes responses induced by DP-RS09-rAd5 and DP/RS09-rAd5 were analyzed. The mucosal secretory IgA level was detected in both nasal and vaginal washes to determine the mucosal immunity. Furthermore,cytokine productions on RAW264.7 cells were tested after preincubation with TLR-4 pathway inhibitors. The results indicated that DEG-PEI could facilitate the intranasal delivery of rAd5 vaccine. Both chemically linked (DP-RS09) and physically mixed RS09 (DP/RS09) could further enhance the mucosal immunity of rAd5 vaccine via TLR-4 pathway. This RS09 adjuvanted DEG-PEI polymer represents a potential intranasal vaccine delivery system and may have a wider application for other viral vectors.
BMC Genomics. 2015 Apr 17;16:307. doi: https://doi.org/10.1186/s12864-015-1511-7
NF-κB and IRF pathways: cross-regulation on target genes promoter level
Background
The NF-κB and IRF transcription factor families are major players in inflammation and antiviral response and act as two major effectors of the innate immune response (IIR). The regulatory mechanisms of activation of these two pathways and their interactions during the IIR are only partially known.
Results
Our in silico findings report that there is cross-regulation between both pathways at the level of gene transcription regulation,mediated by the presence of binding sites for both factors in promoters of genes essential for these pathways. These findings agree with recent experimental data reporting crosstalk between pathways activated by RIG-I and TLR3 receptors in response to pathogens.
Conclusions
We present an extended crosstalk diagram of the IRF – NF-κB pathways. We conclude that members of the NF-κB family may directly impact regulation of IRF family,while IRF members impact regulation of NF-κB family rather indirectly,via other transcription factors such as AP-1 and SP1.
Front. Immunol. 2014 Sep 25;5:461. doi: https://doi.org/10.3389/fimmu.2014.00461
Toll-like receptor signaling pathways
Toll-like receptors (TLRs) play crucial roles in the innate immune system by recognizing pathogen-associated molecular patterns derived from various microbes. TLRs signal through the recruitment of specific adaptor molecules,leading to activation of the transcription factors NF-κB and IRFs,which dictate the outcome of innate immune responses. During the past decade,the precise mechanisms underlying TLR signaling have been clarified by various approaches involving genetic,biochemical,structural,cell biological,and bioinformatics studies. TLR signaling appears to be divergent and to play important roles in many aspects of the innate immune responses to given pathogens. In this review,we describe recent progress in our understanding of TLR signaling regulation and its contributions to host defense.
PLoS ONE. 2012 Feb 20;7(2):e30839. doi: https://doi.org/10.1371/journal.pone.0030839
Synthetic Toll Like Receptor-4 (TLR-4) Agonist Peptides as a Novel Class of Adjuvants
Background
Adjuvants serve as catalysts of the innate immune response by initiating a localized site of inflammation that is mitigated by the interactions between antigens and toll like receptor (TLR) proteins. Currently,the majority of vaccines are formulated with aluminum based adjuvants,which are associated with various side effects. In an effort to develop a new class of adjuvants,agonists of TLR proteins,such as bacterial products,would be natural candidates. Lipopolysaccharide (LPS),a major structural component of gram negative bacteria cell walls,induces the systemic inflammation observed in septic shock by interacting with TLR-4. The use of synthetic peptides of LPS or TLR-4 agonists,which mimic the interaction between TLR-4 and LPS,can potentially regulate cellular signal transduction pathways such that a localized inflammatory response is achieved similar to that generated by adjuvants.
Methodology/Principal Findings
We report the identification and activity of several peptides isolated using phage display combinatorial peptide technology,which functionally mimicked LPS. The activity of the LPS-TLR-4 interaction was assessed by NF-κB nuclear translocation analyses in HEK-BLUE™-4 cells,a cell culture model that expresses only TLR-4,and the murine macrophage cell line,RAW264.7. Furthermore,the LPS peptide mimics were capable of inducing inflammatory cytokine secretion from RAW264.7 cells. Lastly,ELISA analysis of serum from vaccinated BALB/c mice revealed that the LPS peptide mimics act as a functional adjuvant.
Nat Rev Immunol. 2001 Nov 01;1:135–145. https://doi.org/10.1038/35100529
Toll-like receptors and innate immunity
Toll-like receptors have a crucial role in the detection of microbial infection in mammals and insects. In mammals,these receptors have evolved to recognize conserved products unique to microbial metabolism. This specificity allows the Toll proteins to detect the presence of infection and to induce activation of inflammatory and antimicrobial innate immune responses. Recognition of microbial products by Toll-like receptors expressed on dendritic cells triggers functional maturation of dendritic cells and leads to initiation of antigen-specific adaptive immune responses.
J Endotoxin Res. 2000;6(5):401-5. doi: https://doi.org/10.1179/096805100101532243
Role of MD-2 in TLR2- and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes
MD-2 is associated with TLR4 on the cell surface and enables TLR4 to respond to LPS. TLR2 without MD-2 does not respond to pure protein-free endotoxic LPS,ReLPS,and lipid A. MD-2 enables TLR2 to respond to non-activating LPS,ReLPS,and lipid A,and enhances TLR2-mediated responses to Gram-negative and Gram-positive bacteria,protein-containing LPS,peptidoglycan,and lipoteichoic acid. MD-2 enables TLR4 to respond to a wide variety of endotoxic LPS partial structures,Gram-negative bacteria,and Gram-positive lipoteichoic acid,but not to Gram-positive bacteria,peptidoglycan,and lipopeptide. MD-2 physically associates with both TLR4 and TLR2,but the association with TLR2 is weaker than with TLR4. Also,MD-2 and TLR2 and TLR4 enhance each other’s expression. The highest induced genes in human monocytes stimulated with Gram-positive and Gram-negative bacterial cell wall components are chemokine genes,and IL-8 is the highest induced chemokine. Both Gram-positive and Gram-negative bacteria activate TLR2–>MyD88–>IRAK–>TRAF–>NIK–>IKK–>NF–>kappaB signal transduction pathway that induces transcription of the IL-8 gene. Therefore,TLR2 is a functional receptor for both Gram-positive and Gram-negative bacteria and it induces activation of IL-8.
J Exp Med. 1999 Jun 07;189(11):1777–1782. doi: https://doi.org/10.1084/jem.189.11.1777
MD-2,a Molecule that Confers Lipopolysaccharide Responsiveness on Toll-like Receptor 4
Toll-like receptor 4 (TLR4) is a mammalian homologue of Drosophila Toll,a leucine-rich repeat molecule that can trigger innate responses against pathogens. The TLR4 gene has recently been shown to be mutated in C3H/HeJ and C57BL/10ScCr mice,both of which are low responders to lipopolysaccharide (LPS). TLR4 may be a long-sought receptor for LPS. However,transfection of TLR4 does not confer LPS responsiveness on a recipient cell line,suggesting a requirement for an additional molecule. Here,we report that a novel molecule,MD-2,is requisite for LPS signaling of TLR4. MD-2 is physically associated with TLR4 on the cell surface and confers responsiveness to LPS. MD-2 is thus a link between TLR4 and LPS signaling. Identification of this new receptor complex has potential implications for understanding host defense,as well as pathophysiologic,mechanisms.
