[Pyr1]apelin-13 (human) (CAS: 217082-60-5)
Apelin GPCR-Receptor
The Apelin receptor,also known as APJ,APLNR,and AGTRL1,is a member of the class A family of G protein-coupled receptors (GPCRs). It has been identified in various species,including mouse,rat,cow,rhesus macaque,Xenopus laevis,and Danio rerio,and it shares structural similarities with the angiotensin II type l receptor (AT1). However,despite a 54% homology in transmembrane regions with AT1,it does not bind angiotensin II.
Initially designated as an « orphan » GPCR,due to the absence of a known ligand,O’Dowd & al. named it APJ in 19935. Comprising 380 amino acid residues,APJ features the characteristic 7-transmembrane domain structure,typical of a class A GPCR.
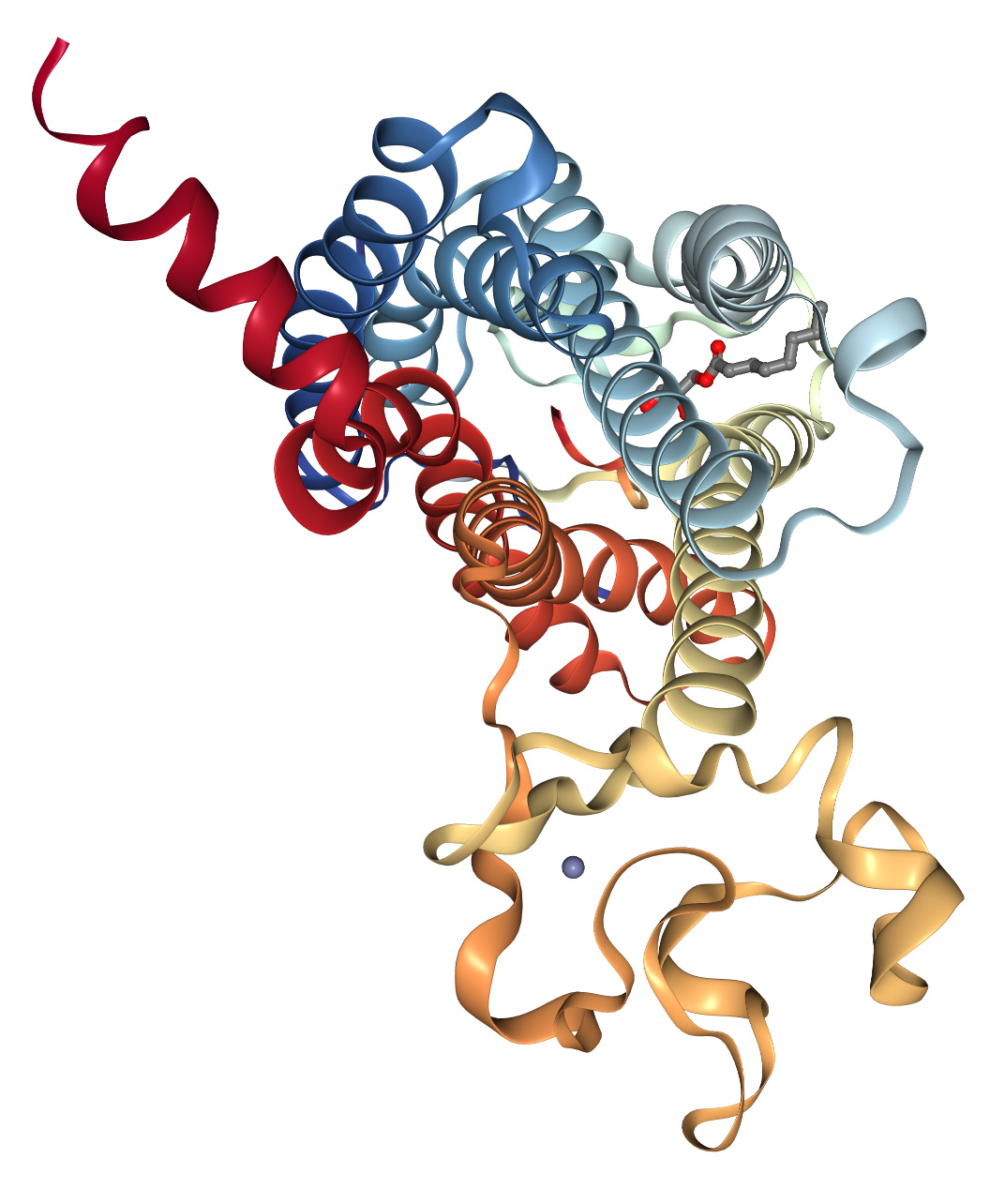
Nowadays,it is known that APLNR depicts physiological roles,including regulation of cardiovascular function,fluid homeostasis,the adipoinsular axis,gastrointestinal and immunomodulatory functions.
Apelin isoforms
Apelin (APLN) endogenous APJ ligand,is a 36-amino acids peptide,initially discovered in bovine stomach extracts. It is expressed and released by cultured adipocytes from a 77-amino acids prepropeptide,whose sequence was identified in human and bovine tissues,with the last 23 residues of the C terminus being identical in mammals.
Cleavage of this preproapelin at specific sites generates different isoforms,including apelin-36,apelin-17,apelin-13,and [Pyr1]apelin-13,which all act as agonists at the apelin receptor and equipotent mediators of vascular tone and cardiac contractility in human tissues in vitro. All of these predicted isoforms have been shown to be present in vivo,but the predominant isoforms in plasma are [Pyr1]apelin-13,apelin-13 and apelin-17,with [Pyr1]apelin-13 and apelin-13. Among these,[Pyr1]apelin-13 and apelin-13 are notably robust activators of apelin receptors in cell lines,while apelin-36 demonstrates remarkable efficacy as an inhibitor of HIV infection in vitro.
[Pyr1]apelin-13 peptide
Although apelin-36 is the most widely expressed APLN isoform,apelin-13 remains the more potent and abundant peptide in blood circulation. Notably,[Pyr1]apelin-13 (CAS: 217082-60-5) is the predominant isoform in human cardiac tissue,showcasing its physiological significance in regulating cardiovascular function.
SB-PEPTIDE is pleased to offer [Pyr1]apelin-13,as well as apelin-36 (see apelin-36 webpage),for further research into their multifaceted functions and therapeutic applications.
Technical specification
 |
Sequence : XRPRLSHKGPMPF (X: pyrGlu) |
 |
MW : 1533.80 g/mol (C69H108N22O16S) |
 |
Purity : > 95% |
 |
Counter-Ion : TFA Salts (see option TFA removal) |
 |
Delivery format : Freeze dried in propylene 2mL microtubes |
 |
Other names : [pGlu1]-Apelin-13 |
 |
Peptide Solubility Guideline |
 |
Bulk peptide quantities available |
Price
| Product catalog | Size | Price € HT | Price $ USD |
| SB311-0.5MG | 0 ,5 mg | 149 | 186 |
| SB311-1MG | 1 mg | 264 | 330 |
References
Nat Struct Mol Biol. 2022 Jul 11;29:688–697. doi: https://doi.org/10.1038/s41594-022-00797-5
Structural insight into apelin receptor-G protein stoichiometry
The technique of cryogenic-electron microscopy (cryo-EM) has revolutionized the field of membrane protein structure and function with a focus on the dominantly observed molecular species. This report describes the structural characterization of a fully active human apelin receptor (APJR) complexed with heterotrimeric G protein observed in both 2:1 and 1:1 stoichiometric ratios. We use cryo-EM single-particle analysis to determine the structural details of both species from the same sample preparation. Protein preparations,in the presence of the endogenous peptide ligand ELA or a synthetic small molecule,both demonstrate these mixed stoichiometric states. Structural differences in G protein engagement between dimeric and monomeric APJR suggest a role for the stoichiometry of G protein-coupled receptor- (GPCR-)G protein coupling on downstream signaling and receptor pharmacology. Furthermore,a small,hydrophobic dimer interface provides a starting framework for additional class A GPCR dimerization studies. Together,these findings uncover a mechanism of versatile regulation through oligomerization by which GPCRs can modulate their signaling.
Front. Endocrinol. Sec. Cardiovascular Endocrinology. 2022 Mar 09;Vol 13. doi: https://doi.org/10.3389/fendo.2022.820002
The Role of Apelin–APJ System in Diabetes and Obesity
Nowadays,diabetes and obesity are two main health-threatening metabolic disorders in the world,which increase the risk for many chronic diseases. Apelin,a peptide hormone,exerts its effect by binding with angiotensin II protein J receptor (APJ) and is considered to be linked with diabetes and obesity. Apelin and its receptor are widely present in the body and are involved in many physiological processes,such as glucose and lipid metabolism,homeostasis,endocrine response to stress,and angiogenesis. In this review,we summarize the literatures on the role of the Apelin–APJ system in diabetes and obesity for a better understanding of the mechanism and function of apelin and its receptor in the pathophysiology of diseases that may contribute to the development of new therapies.
Front. Pharmacol. 2021 Mar 04;12:630548. doi: https://doi.org/10.3389/fphar.2021.630548
The Effects of Apelin and Elabela Ligands on Apelin Receptor Distinct Signaling Profiles
Apelin and Elabela are endogenous peptide ligands for Apelin receptor (APJ),a widely expressed G protein-coupled receptor. They constitute a spatiotemporal dual ligand bsystem to control APJ signal transduction and function. We investigated the effects of Apelin-13,pGlu1 -apelin-13,Apelin-17,Apelin-36,Elabela-21 and Elabela-32 peptides on APJ signal transduction. Whether different ligands are biased to different APJ mediated signal transduction pathways was studied. We observed the different changes of G protein dependent and β-arrestin dependent signaling pathways after APJ was activated by six peptide ligands. We demonstrated that stimulation with APJ ligands resulted in dose-dependent increases in both G protein dependent [cyclic AMP (cAMP),Ca2+ mobilization,and the early phase extracellular related kinase (ERK) activation] and β-arrestin dependent [GRKs,β-arrestin 1,β-arrestin 2,and β2 subunit of the clathrin adaptor AP2] signaling pathways. However,the ligands exhibited distinct signaling profiles. Elabela-32 showed a >1000-fold bias to the β-statin-dependent signaling pathway. These data provide that Apelin-17 was biased toward β-arrestin dependent signaling. Eabela-21 and pGlu1-Apelin-13 exhibited very distinct activities on the G protein dependent pathway. The activity profiles of these ligands could be valuable for the development of drugs with high selectivity for specific APJ downstream signaling pathways.
Front. Neurosci. Sec. Neuroendocrine Science. 2017 Feb 28;Vol 11. doi: https://doi.org/10.3389/fnins.2017.00092
[Pyr1]Apelin-13(1–12) Is a Biologically Active ACE2 Metabolite of the Endogenous Cardiovascular Peptide [Pyr1]Apelin-13
Aims: Apelin is a predicted substrate for ACE2,a novel therapeutic target. Our aim was to demonstrate the endogenous presence of the putative ACE2 product [Pyr1]apelin-13(1–12) in human cardiovascular tissues and to confirm it retains significant biological activity for the apelin receptor in vitro and in vivo. The minimum active apelin fragment was also investigated.
Methods and Results: [Pyr1]apelin-13 incubated with recombinant human ACE2 resulted in de novo generation of [Pyr1]apelin-13(1–12) identified by mass spectrometry. Endogenous [Pyr1]apelin-13(1–12) was detected by immunostaining in human heart and lung localized to the endothelium. Expression was undetectable in lung from patients with pulmonary arterial hypertension. In human heart [Pyr1]apelin-13(1–12) (pKi = 8.04 ± 0.06) and apelin-13(F13A) (pKi = 8.07 ± 0.24) competed with [125I]apelin-13 binding with nanomolar affinity,4-fold lower than for [Pyr1]apelin-13 (pKi = 8.83 ± 0.06) whereas apelin-17 exhibited highest affinity (pKi = 9.63 ± 0.17). The rank order of potency of peptides to inhibit forskolin-stimulated cAMP was apelin-17 (pD2 = 10.31 ± 0.28) > [Pyr1]apelin-13 (pD2 = 9.67 ± 0.04) ≥ apelin-13(F13A) (pD2 = 9.54 ± 0.05) > [Pyr1]apelin-13(1–12) (pD2 = 9.30 ± 0.06). The truncated peptide apelin-13(R10M) retained nanomolar potency (pD2 = 8.70 ± 0.04) but shorter fragments exhibited low micromolar potency. In a β-arrestin recruitment assay the rank order of potency was apelin-17 (pD2 = 10.26 ± 0.09) >> [Pyr1]apelin-13 (pD2 = 8.43 ± 0.08) > apelin-13(R10M) (pD2 = 8.26 ± 0.17) > apelin-13(F13A) (pD2 = 7.98 ± 0.04) ≥ [Pyr1]apelin-13(1–12) (pD2 = 7.84 ± 0.06) >> shorter fragments (pD2 < 6). [Pyr11apelin-13(1–12) and apelin-13(F13A) contracted human saphenous vein with similar sub-nanomolar potencies and [Pyr1]apelin-13(1–12) was a potent inotrope in paced mouse right ventricle and human atria. [Pyr1]apelin-13(1–12) elicited a dose-dependent decrease in blood pressure in anesthetized rat and dose-dependent increase in forearm blood flow in human volunteers.
Conclusions: We provide evidence that ACE2 cleaves [Pyr1]apelin-13 to [Pyr1]apelin-13(1–12) and this cleavage product is expressed in human cardiovascular tissues. We have demonstrated biological activity of [Pyr1]apelin-13(1–12) at the human and rodent apelin receptor in vitro and in vivo. Our data show that reported enhanced ACE2 activity in cardiovascular disease should not significantly compromise the beneficial effects of apelin based therapies for example in PAH.
Pharmacol Rev. 2010 Sep;62(3):331-42. doi: https://doi.org/10.1124/pr.110.002949
International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature,distribution,pharmacology,and function
A gene encoding a novel class a G-protein-coupled receptor was discovered in 1993 by homology cloning and was called APJ. It was designated an « orphan » receptor until 1998,when its endogenous ligand was identified and named apelin (for APJ endogenous ligand). Since this pairing,both apelin and its receptor have been found to have a widespread distribution in both the central nervous system and the periphery. A number of physiological and pathophysiological roles for the receptor have emerged,including regulation of cardiovascular function,fluid homeostasis,and the adipoinsular axis. This review outlines the official International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification nomenclature,designating the receptor protein as the apelin receptor,together with current knowledge of its pharmacology,distribution,and functions.
Gene. 1993 Dec 22;136(1-2):355-60. doi: https://doi.org/10.1016/0378-1119(93)90495-o
5A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11
We report the cloning of a gene,intronless in its coding region,which we have named APJ. This gene was cloned using the polymerase chain reaction (PCR),with a set of primers designed on the basis of the conservation that members of G protein-coupled receptors (GPCR) have in their transmembrane (TM) regions. The putative receptor protein,APJ,shares closest identity to the angiotensin receptor (AT1) ranging from 40 to 50% in the hydrophobic TM regions of these receptors. The transcripts for this gene were detected in many regions of the brain. PCR analysis of somatic cell lines found APJ-related sequences to be only present on chromosome 11,and high-resolution mapping by fluorescence in situ hybridization (FISH) sublocalized APJ on band q12.
