Peptides labelled with affinity tags
SB-PEPTIDE can conjugate and functionnalize peptides with many different compounds including affinity tags. Affinity-tags can be biotin, maleimide, alkyne and azide function or thiol functions. These functions serve for bioconjugations, in this case between a peptide and something else (another peptide, protein, a surface, a fluorescent dye, a DNA…).
Affinity tagged peptides applications
- Purification by affinity using appropriate columns
- Labeling for detection assay using a fluorescent streptavidine tag or an antibody anti-biotin
- Immobilization on a solid support
Affinity tags available
His-tag peptides :
SB-PEPTIDE can synthesize peptides with several histidines at C-ter or N-ter to serve as His-tag.
Biotinylated-peptides:
Biotin, also known as vitamin B8 or B7 is water soluble. Biotin is widely used in bioconjugation thanks to its high affinity for streptavidine a 52,8 kDa protein with a Kd in the range of 10(−14) mol/L. Biotinylated-peptide is widely used for ELISA or pull-down assays. As biotin-streptavidin forms a large complex (>50kDa) compared to a few kilodaltons peptide, we recommend especially for fluorescent labeling to opt-in for another strategy. SB-PEPTIDE can conjugate specifically one biotin per custom synthetic peptides in N-ter, C-ter or within the sequence.
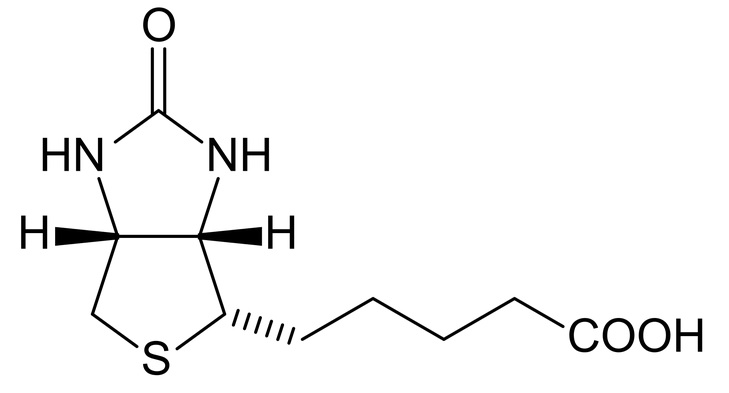
Maleimide-peptide:
SB-PEPTIDE can incorporate a maleimide function at N-ter, C-ter or inside the sequence. Maleimide can react with thio functions of cysteine to form a stable carbon-sulfur bond making it very strong and specific. Thiol-maleimide conjugation is widely used in bioconjugation thanks to its simplicity and attractive conjugation yield.
Azide/Alkyne-peptide:
Several azido and alkyne functions are available to prepare azido-peptide or alkyne-peptide suitable for click-chemistry. Click-chemistry is a reaction used in bioconjugation to specifically conjugate one compound azide-functionalized with another compound alkyne-functionalized. The classic click reaction is the copper-catalyzed reaction of an azide with an alkyne to form a 5-membered heteroatom ring: a Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). Alkyne-peptides or azide-peptides are valuable approaches to consider for in-solution labeling when amide bonds or thiol-maleimide conjugations are not possible due to amino acids contained in the sequence.
Thiol-peptide:
Thiol-peptides are peptides containing a thiol function. Thiol function can be incorporated by adding a cysteine during the synthesis or other thiol existing thiolated compounds. Thiol-peptides are widely used for maleimide conjugation such as peptide-BSA or peptide KLH conjugation or for peptide attachment to gold surface (i.e. SPR).
Others upon request
Tags can be placed at various positions of the peptide such as N-ter, C-ter or within the peptide. N-terminal conjugation is generally the easier way, side chain labeling can also be performed. These functions are also availble for PNA custom synthesis.
Custom peptide synthesis
SB-PEPTIDE can custom synthesize peptides and functionalize them with affinity tags. Please get in touch for project evaluation.
- Amyloid Peptides
- Acetyl-ccbeta
- beta-Amyloid (1-11) Human
- beta-Amyloid (1-12) Human
- beta-Amyloid (1-14)
- beta-Amyloid (1-15) human
- beta-Amyloid (1-16) Human
- beta-Amyloid (1-17) Human
- beta-Amyloid (1-28) human
- beta-Amyloid (1-40) Human
- beta-Amyloid (1-6)-GGC Human
- beta-Amyloid (10-20) Human
- beta-Amyloid (11-20) Human
- Beta-Amyloid (12-20)
- beta-Amyloid (35-25)
- RNase A (77-82) Amyloidogenic peptide
- SEN 304
- Tau (195-209) Light
- Tau Peptide (45-73)
- Antimicrobial Peptides - AMP
- Abaecin
- Acetyl-Adhesin
- AcrAP1
- AcrAP1a
- AcrAP2
- AcrAP2a
- Alloferon 1
- Alloferon 2
- Alyteserin-1a
- Alyteserin-1b
- Alyteserin-1c
- Alyteserin-1d
- Alyteserin-2a
- Alyteserin-2b
- Alyteserin-2c
- Alyteserin-2Mb
- Anoplin
- Apidaecin IB
- B8R (20 – 27)
- Balteatide
- BMAP-18
- BMAP-18 (truncated)
- Buforin II
- Cecropin A
- Codesane
- CRAMP (1-39)
- CRAMP (6-39)
- CRAMP-18
- Cyclic L27-11
- Cycloviolacin O2 peptide
- Epinecidin-1
- FdM
- Feleucin-B01
- Gag (18-26) [Human immunodeficiency virus type 1] acetyl/amide
- Gag peptide [Simian immunodeficiency virus]
- Gag protein (181-189) acetyl/amide [Simian immunodeficiency virus]
- GLK-19
- hBD-2 peptide – human Beta Defensin 2
- HEL46-61
- Hepcidin-25 (LEAP-1 peptide)
- Histatin-5
- Histatin-8
- Hp1404
- Human Lysozyme (107-115)
- IDR-1
- Indolicidin
- Influenza Virus Nucleoprotein (311 – 325)
- KDAMP
- LL-13-37
- LL-17-29
- LL-17-32
- LL-37 amide
- LL-37 fragment (24-29)
- LL-37 fragment (30-34)
- LL-37 fragment (30-37)
- LL-37 peptide (CAP-18)
- Macropin 1
- Macropin 2
- Magainin II
- Magainin-1
- MALT1 substrate
- Mastoparan
- Mastoparan peptide
- mBD3 peptide – mouse Beta Defensin 3
- MP196
- N-formylated PSMalpha2
- N-formylated PSMalpha3
- P2-Hp-1935
- PA protein (Influenza A virus)
- PAF19
- PAF26
- Pantinin-1
- Pantinin-2
- Pantinin-3
- Pap12-6
- Parasin I
- Pseudin-2
- Sapecin B
- SARS-CoV-2 NSP13 (221-235)
- SARS-CoV-2 NSP13 (226-240)
- SARS-CoV-2 NSP13 (231-245)
- SARS-CoV-2 NSP13 (236-250)
- SARS-CoV-2 NSP13 (241-255)
- SARS-CoV-2 NSP13 (246-260)
- SARS-CoV-2 NSP13 (321-335)
- SARS-CoV-2 NSP13 (326-340)
- SARS-CoV-2 NSP13 (336-350)
- SARS-CoV-2 NSP13 (421-435)
- SARS-CoV-2 NSP13 (426-440)
- SARS-CoV-2 NSP13 (466-480)
- SARS-CoV-2 NSP13 (476-490)
- SARS-CoV-2 NSP13 (551-565)
- SARS-CoV-2 NSP13 (556-570)
- SARS-CoV-2 NSP13 (576-590)
- SARS-CoV-2 NSP13 (581-595)
- SARS-CoV-2 NSP7 (1-15)
- SARS-CoV-2 NSP7 (21-35)
- SARS-CoV-2 NSP7 (31-45)
- SARS-CoV-2 NSP7 (46-60)
- SARS-CoV-2 NSP7 (51-65)
- SARS-CoV-2 NSP7 (6-20)
- SARS-CoV-2 Nucleoprotein (1-17)
- SARS-CoV-2 Nucleoprotein (104-121)
- SARS-CoV-2 Nucleoprotein (126-140)
- SARS-CoV-2 Nucleoprotein (266-280)
- SARS-CoV-2 Nucleoprotein (271-285)
- SARS-CoV-2 Nucleoprotein (321-335)
- SARS-CoV-2 Nucleoprotein (331-345)
- SARS-CoV-2 Nucleoprotein (341-355)
- SARS-CoV-2 Nucleoprotein (346-360)
- SARS-CoV-2 Nucleoprotein (351-365)
- SARS-CoV-2 Nucleoprotein (353-370)
- SARS-CoV-2 Nucleoprotein (356-370)
- SARS-CoV-2 Nucleoprotein (51-65)
- SARS-CoV-2 Nucleoprotein (61-75)
- SARS-CoV-2 Nucleoprotein (86-100)
- SARS-CoV-2 Nucleoprotein 1 (56-70)
- SARS-CoV-2 Nucleoprotein 2 (326-340)
- SARS-CoV-2 ORF7a-10 (69-86)
- SARS-CoV-2 Spike (1192-1200)
- SARS-CoV-2 Spike (1196-1205)
- SARS-CoV-2 Spike (1197-1206)
- SARS-CoV-2 Spike (236-250)
- SARS-CoV-2 Spike (757-765)
- SARS-CoV-2 Spike (781-795)
- SARS-CoV-2 Spike (975-983)
- SARS-CoV-2 Spike (975-989)
- SARS-CoV-2 Spike (991-1000)
- SARS-CoV-2 Spike (996-1004)
- SARS-CoV-2 Spike (999-1007)
- SARS-inhibitor 4
- Scrambled LL-37 peptide
- Secretin (rat)
- Sendai Virus nucleoprotein (324-332)
- T22 peptide
- Temporin A
- Temporin L
- Tritrpticin
- VP4 (449-454) Nora virus
- VP4 (93-101) Nora virus
- [5-FAM]-LL-37
- [Cys]-Influenza Virus Nucleoprotein (311 – 325)
- Biotin Labeled
- BCL-6 corepressor Human (BCOR) (498-514) C-terminal Biotin
- beta-Amyloid (1-10) Biotin
- beta-Amyloid (1-11) Biotin
- beta-Amyloid (1-12) Biotin
- beta-Amyloid (1-13) Biotin
- beta-Amyloid (1-14) Biotin
- Biotin Apolipoprotein A-I (APOA1)(86-101)
- Biotin BRC4 (1517-1551)
- Biotin gliadin-derived peptide
- Biotin HER-2 substrate peptide
- Biotin phosphorylated CDK7 (157-169)
- Biotin phosphorylated JAK1 substrate peptide
- Biotin SBP1
- Biotin SBP2
- Biotin Steroid Receptor Coactivator-1 (SRC-1) (676-700)
- Biotin Substance P
- Biotin TAT (48-60)
- Biotin-aMp3
- biotin-aMptD
- Biotin-Axltide Peptide substrate
- Biotin-beta-Amyloid (1-15) human
- Biotin-Desmoglein-3 DSG3 (50-79)
- Biotin-GLP-1 (7-36)
- Biotin-Histone H3 (14-34) K23Me3
- Biotin-Histone H3 (14-34) pT22 K23Me3
- Biotin-Influenza A NP (147-155) (H-2Kd)
- Biotin-Jak2 substrate
- Biotin-LPETAG N-terminal Sortagging
- Biotin-LPETGG N-terminal Sortagging
- Biotin-Nrf2 (69-84)
- Biotin-PEG2-Claudin-3
- Biotin-PEG2-Claudin-6
- Biotin-PEG2-Claudin-9
- Biotin-TAT (47-57)
- Biotin-β Amyloid (1-42) Human
- Biotinylated L57
- C-Terminal Sortagging-AAA-[Lys(Biotin]
- C-Terminal Sortagging-[Lys(Biotin]
- Galanin (2-13)-Biotin
- Galanin (3-13)-Biotin
- Histone H2A (1-20)-GGK(Biotin)
- Histone H3 (1-20)
- Histone H3 (1-20)-[S]-Biotin
- Histone H3 (1-22) K4Me3-Biotin
- Histone H3 (1-22) K9Me1-Biotin
- Histone H3 (10-29)-Biotin
- Histone H3 (20-39)-Biotin
- MHC class II antigen E alpha (52-68)-Biotin
- Pyroglutamyl beta-Amyloid (4-14) Biotin
- [Biotin]-GLP-1
- Cancer Peptides
- A6 peptide
- AD01 N-terminal Q
- ARF peptide
- Bak BH3
- Bevacizumab Light chain
- Bid BH3 Peptide
- BIM 187
- Bim BH3, Peptide IV
- Braftide
- C7
- CooP
- Cyclo(CLLFVY)
- D-Arg PEP
- dodecapeptide AR71
- DOTA-(Tyr3)-octreotate Acetate Salt
- FAM49B (190-198) Mouse
- FFW
- FREG peptide
- GRGD-acid
- HIF-1 α (556-574)
- Infliximab Heavy chain (46-60)
- PEN-FFW
- Proapoptotic Peptide KLA
- RAGE antagonist peptide
- Rituximab Light chain (41-55)
- RKOpep
- XL 13m
- YSA acid
- [Tyr0]-Apelin-13
- Cell Penetrating Peptides - CPP
- (Arg)9 peptide
- (RFR)4XB
- 3xFlag [DYKDDDDK]
- Acetyl-Arg9
- Angiopep 2
- Antennapedia peptide
- Antennapedia peptide amide
- Antennapedia peptide Arg
- Azhx-Penetratin
- C105Y
- CPP9
- Cys(Npys)-Antennapedia peptide amide
- DYKDDDDK FLAG peptide
- Flexible Glycine Linker (3xGGGGS)
- L17E
- MART-1 (26-35)
- MHV EP™
- N3-His tag
- Penetratin peptide
- Pep – 1 – Chariot
- PR9
- RAG8
- Secretoneurin Mouse Rat
- T peptide
- TAT (47-57) peptide
- TfR targeting sequence
- [5-FAM]-(RXR)4XB
- Click Peptides
- COVID-19 Peptides
- ACE2 – Angiotensin-Converting enzyme 2 – peptide library
- Biotin-SARS-CoV-2 Spike RBD 319-335 peptide
- Biotin-SARS-CoV-2 Spike RBD 336-347 peptide
- Biotin-SARS-CoV-2 Spike RBD 348-357 peptide
- Biotin-SARS-CoV-2 Spike RBD 352-365 peptide
- Biotin-SARS-CoV-2 Spike RBD 371-394 peptide
- Biotin-SARS-CoV-2 Spike RBD 395-430 peptide
- Biotin-SARS-CoV-2 Spike RBD 513-520 peptide
- Biotin-SARS-CoV-2 Spike RBD 523-541 peptide
- Biotin-SARS-CoV-2 Spike RBM 438-458 peptide
- Biotin-SARS-CoV-2 Spike RBM 450-473 peptide
- Biotin-SARS-CoV-2 Spike RBM 480-496 peptide
- Biotin-SARS-CoV-2 Spike RBM 500-509 peptide
- CoV Main Protease (Mpro) Substrate
- SARS CoV-2 Spike (S) protein – Peptide library
- SARS-CoV 3C-like protease (3CLpro) substrate (C-terminal KK-acid)
- SARS-CoV-2 Membrane protein (141-158)
- SARS-CoV-2 Membrane protein (172-188)
- SARS-CoV-2 NSP7 (26-40)
- SARS-CoV-2 Nucleocapsid (N) protein – Peptide library
- SARS-CoV-2 Nucleoprotein 2 (261-275)
- SARS-CoV-2 ORF3a (26-40)
- SARS-CoV-2 Spike RBD 319-335 peptide
- SARS-CoV-2 Spike RBD 336-347 peptide
- SARS-CoV-2 Spike RBD 348-357 peptide
- SARS-CoV-2 Spike RBD 352-365 peptide
- SARS-CoV-2 Spike RBD 371-394 peptide
- SARS-CoV-2 Spike RBD 395-430 peptide
- SARS-CoV-2 Spike RBD 513-520 peptide
- SARS-CoV-2 Spike RBD 523-541 peptide
- SARS-CoV-2 Spike RBM 438-458 peptide
- SARS-CoV-2 Spike RBM 450-473 peptide
- SARS-CoV-2 Spike RBM 480-496 peptide
- SARS-CoV-2 Spike RBM 500-509 peptide
- Spike Protein (SARS-CoV-2) Peptide Pool
- UCI-1
- Variants package of SARS CoV-2 Spike (S) protein mutation – Peptide library
- Fluorescent Peptides
- (Cbz-LGR)2-[Rh110]
- (N-Cbz-Nle-KRR)2-[Rh110]
- (PFR)2-[Rh110]
- (Tos-GFHR)2-[Rh110]
- (Tos-GPR)2-[Rh110]
- (Tos-YASR)2-[Rh110]
- 5-FAM-Fz7-21
- AAA-C(AF647) C-Terminal Sortagging
- Ac-Arg-Gly-Lys(Ac)-AMC
- Ac-GPLD-[Rh110]-[D-Pro]
- Ac-RGK-[AMC]
- Ac-RLR-[AMC] Proteasome Substrate
- Ac-RLR-[Rh110]-[D-Pro]
- Acetyl-Alpha-2-antiplasmin-[AF680]
- Acetyl-Histone H4 (1-21) K5Ac, K8Ac, K12Ac, K16Ac-GG-[Lys(5-FAM)]
- Acetyl-Histone H4 (1-23) K16Ac-GG-[Lys(5-FAM)]
- Acetyl-Histone H4 (1-23)-GG-[Lys(5-FAM)]
- acfTAT
- ACTH (1-24) -[5-FAM]
- AF488 6xHis Tag
- AF488 Insulin
- AF488 Plectin-1-targeting peptide
- AF647 RGD peptide
- C-terminal Sortagging-[Cys(AF488)]
- C-terminal Sortagging-[Cys(AF680)] acid
- C-terminal Sortagging-[Cys(AF680)] amide
- C-terminal Sortagging-[Cys(Sulfocyanine3)]
- C-terminal Sortagging-[Cys(Sulfocyanine5)]
- C-terminal Sortagging-[Cys(Sulfocyanine7)]
- Cathepsin G FRET substrate [5-FAM]/[6-TAMRA]
- Cys(BDP630/650)-Galanin (1-30) Human
- DNA damage-binding protein 2 (DDB2)-[Cys(AF647)]-amide
- ERAAP substrate Ep
- Exendin-4 [Lys(AF647)]
- FLAG tag (Cy3B)
- Fluorescein HLA-A*02:01 HBV core (18-27)
- Formyl-MLF-[Cys(AF488)]
- GGG-C(AF647) C-Terminal Sortagging
- GGG-[K(5-TAMRA)] C-terminal Sortagging
- Ghrelin-[Cys(AF647)] Human
- GLP-1 (7-36) [Cys(Sulfocyanine5)]
- Glucagon (1-29)-[Cys(Cy5)]
- Glucagon (1-29)-[Lys(AF647)]
- GRGD-[Cys(AF647)]
- H-Met-Gly-Pro-[AMC].HCl
- HCV NS3 protease FRET substrate
- Histone H3 (1-15) K4Me3, K9Ac, pS10
- Histone H3 (1-20) K4Me2-GG-[Lys(5-FAM)]
- Histone H3 (1-20) K4Me3, K9Ac, pS10-GG-[Cys(Aurora™ Fluor 647)]
- Histone H3 (1-20) K4Me3, K9Ac, pS10-GG-[Lys(5-FAM)]
- Histone H3 (1-20) K4Me3, K9Ac-GG-[Lys(5-FAM)]
- Histone H3 (1-20) K4Me3, pS10-GG-[Lys(5-FAM)]
- Histone H3 (1-20) K4Me3-GG-[Cys(Aurora™ Fluor 647)]
- Histone H3 (1-20) K4Me3-GG-[Lys(5-FAM)]
- Histone H3 (1-20) pT3, K4Me3-GG-[Lys(5-FAM)]
- Histone H3 (1-20)-GG-[Cys(Aurora™ Fluor 647)]
- Histone H3 (1-20)-GG-[Lys(5-FAM)]
- Histone H3-GG-[Cys(5-FAM)]-amide
- Insulysin FRET substrate [Mca]/[Dnp]
- KHLF-[AMC]
- LasB FRET substrate
- Leu-AFC.HCl
- Melittin [Cy5]
- PFR-[AMC]
- Plasmin-[AMC] substrate
- Ser3-n-octanoyl Ghrelin-[Cys(AF647)] Human
- SMAC/DIABLO [Lys(5-FAM)]
- SMAC/DIABLO-[Cys(AF647)]
- SMRT peptide-(Cys[AF633])
- Suc-LLVY-[AMC]
- Suc-LLVY-[Rh110]-[D-Pro]
- [β-Ala]-[Lys(5-TAMRA)]-acid
- [β-Ala]-[Lys(AMCA)]-acid
- [5-FAM] Antennapedia peptide amide
- [5-FAM] EGFR/kinKDR peptide substrate
- [5-FAM] Histone H3 (1-14) K4Me3
- [5-FAM] Kemptide
- [5-FAM]-(KFF)3K
- [5-FAM]-(RFR)4XB
- [5-FAM]-Arg9
- [5-FAM]-beta-Amyloid (1-15) Human
- [5-FAM]-C7
- [5-FAM]-CADY
- [5-FAM]-Collagen alpha-1(I)-(5-TAMRA)
- [5-FAM]-CRAMP (6-39)
- [5-FAM]-EB1
- [5-FAM]-ERKtide
- [5-FAM]-Galanin (1-30) Human
- [5-FAM]-GLP-1
- [5-FAM]-GLP-1 (7-36)
- [5-FAM]-IFN-γ receptor (pTyr) peptide
- [5-FAM]-M918
- [5-FAM]-MAP
- [5-FAM]-MPG∆NLS
- [5-FAM]-PR9
- [5-FAM]-PTH (1-34)
- [5-FAM]-pVec
- [5-FAM]-RGD peptide
- [5-FAM]-RKOpep
- [5-FAM]-RPKPQQFFGLM-NH2
- [5-FAM]-SRC Substrate Peptide
- [5-FAM]-TAT
- [5-FAM]-TAT (47-57) amide
- [5-FAM]-Tp10
- [5-FAM]-Tyr-Ahx-Ser-Asp-Lys-Pro-acid
- [5-FAM]-Val
- [5-FAM]-VGB4
- [5-FAM]/[Lys(Dabcyl)]-CoV Main Protease (Mpro) Substrate
- [5-FAM]/[Lys(Dnp)]-SARS-CoV-2 S1/S2
- [5-TAMRA] Galanin, Human
- [5-TAMRA]-ATIA agonist [sar1, Ile4, Ile8]
- [5-TAMRA]-Galanin (1-30) Human
- [5-TAMRA]-LPETAG N-terminal Sortagging
- [5-TAMRA]-LPETGG N-terminal Sortagging
- [5-TAMRA]/[Lys(BHQ-2)] Ubiquitin
- [5-TAMRA]/[Lys(BHQ-2)]-CoV Main Protease (Mpro) Substrate
- [6-FAM]-Arg8
- [Atto655]-LifeAct (Abp140 1-17)
- [Aurora™ Fluor 647]-RGD peptide
- [Azhx]-[Lys(Mca)]-P11-8
- [BDP630/650]3-halphaCGRP (calcitonin gene-related peptide)
- [Cy3B]-LifeAct (Abp140 1-17)
- [Cys(AF488)]-Penetratin
- [Cys(AF647)]-Jak2/3 substrate
- [DABCYL]/[Glu(EDANS)] SARS-CoV-2 3C-like protease (3CLpro) substrate
- [FITC]-Ahx-(KKEEE)3K carrier peptide
- [FITC]-C7
- [FITC]-pCREB (127-134) substrate
- [MCA]/[Lys(Dnp)]-CoV Main Protease (Mpro) Substrate
- [Rhodamine Green]-LifeAct (Abp140 1-17)
- [Sulfo-Cyanine3]-LifeAct (Abp140 1-17)
- [Sulfo-Cyanine5]-Val-Pro-Valp(OPh)2
- [TAMRA]-beta-Amyloid (1-15) Human
- ™PRSS4 (199-207) fluorogenic peptide
- GPCR Modulators
- Growth Factors and Cytokines
- Boc-Val-Pro-Arg-AMC
- CHKtide
- CSK substrate
- EGFR (963-975)
- EGFR/kinKDR peptide substrate
- HSP70/DnaK Substrate Peptide
- IRS-1 substrate
- LHRH
- N-methylated ERAP1substrate
- Phosphorylated CHKtide
- Phosphorylated Sakamototide
- PKA Substrate
- Renin substrate
- Sakamototide
- SAMS peptide
- Somatostatin 14 (human, rat, mouse, pig, chicken, frog)
- SRC Substrate Peptide
- Hematology Related Peptides
- Histone Peptides
- Acetyl-Histone H4 (1-21)
- H4 peptide (16-23)
- Histone H1 derived peptide
- Histone H2A (1-20)
- Histone H2A (78-86)
- Histone H3 (1-18)
- Histone H3 (1-20) K4Me3
- Histone H3 (1-20) K4Me3, K9Ac, pS10-GG-Biotin
- Histone H3 (1-21)
- Histone H3 (1-21) K4ac
- Histone H3 (1-21) K4Me2
- Histone H3 (1-21) K4Me3
- Histone H3 (1-21) K9Me2
- Histone H3 (1-22)
- Histone H3 (1-8)
- Histone H3 (20-36) K27Me3
- Histone H3 (22-30) K27Me3
- Histone H3 (30-41) K36Me2
- Histone H3 (32-38) K36Me2
- Histone H3 (32-47)
- Histone H3-GGC-amide
- Histone H3.2 (1-44)
- Histone H3.3 (1-44)
- Histone H4 (1-21)
- Histone H4 (1-21) R3Me1
- Histone H4 (1-21) R3Me2
- Histone H4 (1-23)
- Osteogenic Growth Peptide (OGP)
- SETD8 Peptide
- Hormones
- ACTH (7-39) human
- Alexamorelin
- Alpha mating factor – WHWLQLKPGQPMY
- ANP (1-23)
- ANP (13-26)
- ANP (7-20)
- ANP (7-23)
- ANP (9-22)
- ANP 1-28 Human
- BNP-32 human
- Bradykinin
- Calcitonin, human – Agonist of the calcitonin receptor CTR
- Calcitonin, Rat
- Calcitonin, Salmon
- CCL2 (MCP-1)
- Exendin 3 (9-39) amide
- Exendin 4 (4-39)
- Exendin 4 – Potent GLP-1R agonist
- Gastrin Releasing Peptide, human
- GIP (1-42)-[C] human
- GIP (Pro 3)
- GIP, human
- GLP-1 (1-37)
- GLP-1 (7-36) amide
- GLP-1 (9-36) amide
- Glucagon (3-29)
- Glucagon like-peptide-2 (GLP-2)
- GPS1573
- GRP (14-27), human, porcine
- h-Chemerin-9 (149-157)
- Insulin beta Chain Peptide (15 – 23)
- Isotocin
- Liraglutide
- Motilin (1-10)
- Motilin (1-12)
- Motilin (human, porcine)
- Oxytocin
- Pro-BNP (47-76)
- Protirelin – Thyrotropin-releasing hormone (TRH) (CAS: 24305-27-9)
- PTH (1-13) Human
- PTH (1-34) human
- PTH (13-34) Human
- RANTES (CCL5)
- [Glu2]-TRH peptide (CAS: 85541-78-2)
- Immunology - Antigens/Epitotes/Pools/Librairies
- 2-Furoyl-LIGRL-amide
- 4-Fluorobenzoyl-A20FMDV2
- A*02:01/Human Survivin (96-104) peptide – LTLGEFLKL
- A*02:01/Human Survivin/SurA2.M (LMLGEFLKL) peptide
- AAV8 capsid protein
- Acetyl-HIV-1 reverse transcriptase (A2-YI9)
- Acetyl-TGF-beta 2-LAPbeta (259- 269)
- AF12198
- AH1 Sequence (6-14)
- AIP-I
- AIP-II
- AIP-III
- AIP-IV
- Allergen Ara h 1 (560-572)
- alpha-Gliadin (31 – 43)
- Annexin A1 (2-12)
- Ara h 2 (147-155) peanut Allergen
- Ara h 3 (278-284) peanut Allergen
- Ara h 6 (120-131) peanut Allergen
- Ara h1 (555-577) peanut Allergen
- AYPGFK Protease-Activated Receptor-4 (PAR-4)
- B-peptide
- BAM (8-22)
- BAT3 (340-347), human
- BDC2.5 mimotope 1040-51
- Biotin-FluM1 (58-66) peptide
- Biotin-MAGE-A1 (278-286) peptide
- Biotin-MAGE-A2 (157-166) peptide
- Biotin-NY-ESO-1 (157-165) C165V peptide
- Biotin-Ova (323-339) peptide
- Biotin-PADRE peptide AKFVAAWTLKAAA
- C5A
- CD20 (188-196) peptide – SLFLGILSV
- CEF (HLA Class I Control) Peptide Pool
- CEFT Control Peptide Pool (HLA Class I Control)
- Cilengitide (Linear)
- CMV IE-1 (213-225)
- CMV pp65 (113-121) peptide – VYALPLKML
- CMV pp65 (120-129) peptide – MLNIPSINV
- CMV pp65 (415-429) (HLA-B7)
- CMV pp65 (485-500)
- CMV pp65 (495-503) (HLA-A2)
- CMV pp65 (511-525)(HLA-B44)
- Compstatin
- Cyclo(-RGDyK)
- Cyclo(RGDfK)
- EBV BMLF1 (280-288) (HLA-A2)
- EBV BMLF1 (280-288) peptide – GLCTLVAML
- EBV BNRF1 (1238-1252)
- EBV BRLF1 (134-142) (HLA-A11)
- EBV BRLF1 (148-156) (HLA-A3)
- EBV BRLF1 (28-37) (HLA-A24)
- EBV BZLF1 (190-197) (HLA-B8)
- EBV BZLF1 (40-48) (HLA-E)
- EBV EBNA3A (158-166) (HLA-B8)
- EBV EBNA3A (325-333) (HLA-B8)
- EBV EBNA3A (379-387) (HLA-B7)
- EBV EBNA3A (458-466) (HLA-B35)
- EBV EBNA3A (603-611) (HLA-A3)
- EBV EBNA3B (416-424) (HLA-A11)
- EBV EBNA3C (258-266) (HLA-B27)
- EBV EBNA3C (281-290) (HLA-B44)
- ELA Elabela/Toddler-32
- Fibrinogen, b43-63
- Fibrinopeptide
- Flu-HA-B (306-318) MHC II DRB1*01:01
- FluM1 (58-66) peptide – GILGFVFTL
- GHK tripeptide
- gp100 (25-33) epitope – KVPRNQDWL
- gp100 (280-288) A288V HLA-A*0201 antigen peptide – YLEPGPVTV
- GP33 (1-9)
- gp96-II
- Haemagglutinin (HA) peptide YPYDVPDYA
- HCV NS5B (2594-2602) peptide – ALYDVVTKL
- Her-2/neu (85-94) peptide – LIAHNQVRQV
- HIV-1 p17 Gag (77-85) peptide – SLYNTVATL
- HIV-1 reverse transcriptase (A2-YI9)
- HLA-A*02:01 HBV core (18-27)
- HLA-A*02:01 NY-ESO-1 (157-165)
- HLA-A*02:01 Polymerase (400-408)
- HLA-A*02:01 Polymerase (417-425)
- HLA-DRB1*1501 peptide
- HPV E7 protein (49-57)
- HPV16 E7 (86-93)
- HS1 protein (160-168)
- hsBCL9CT-24
- Human PD – L1 inhibitor V
- I-A(g7) BDC2.5 mimotope
- IDR 1002
- IFNB1 (118-132) Human
- IL-33 peptide
- Influenza A HA (306-318)
- Influenza A NP (265-273) (HLA-A3)
- Influenza A NP (380-388) (HLA-B8)
- Influenza A NP (383-391) (HLA-B27)
- Influenza A NP (44-52) (HLA-A1)
- Influenza A NP (91-99) (HLA-A68)
- Influenza A PB1 (591-599) (HLA-A1)
- Interleukin-27 subunit beta (22-30)
- KD20 peptide
- MAGE-A p248V9 peptide – YLEYRQVPV
- MAGE-A p248V9 scrambled (RQYVELPYV)
- MAGE-A1 (278-286) peptide – KVLEYVIKV
- MAGE-A1 (278-286) scrambled (ELIVKVYKV)
- MAGE-A2 (157-166) peptide – YLQLVFGIEV
- MAGE-A2 (157-166) scrambled (VLVYFQEIGL)
- MAGE-A3 (112-120) peptide – KVAELVHFL
- MAGE-A3 (FLWGPRALV)
- MAGE-A3 (IMPKAGLLI)
- MBP-B MHC II DRB1*15:01 (84-102)
- Melan-A (26-35) A27L peptide – ELAGIGILTV
- Melan-A (26-35) peptide – EAAGIGILTV – CAS: 156251-01-3
- Melan-A (26-35) scrambled (AIEIAGGLTV)
- MHC binding peptides prediction
- MOG (34-56) Human amide
- MOG (35-51) cit46 human
- MOG (35-55) amide Mouse, Rat
- MUC1 (12-20) peptide – LLLLTVLTV
- Myoglobin 137-148 MHC II DRB1*03:01
- Nangibotide
- NY-ESO-1 (123-137) DRB1*04:01 peptide – LKEFTVSGNILTIRL
- NY-ESO-1 (157-165) C165V peptide – SLLMWITQV
- NY-ESO-1 (157-165) C165V scrambled (MSILWQLVT)
- NY-ESO-1 (157-165) peptide – SLLMWITQC
- ORF65 (131-140) [Murid herpesvirus 4]
- OVA (250-264)
- OVA (251-264)
- OVA (323 – 339) amide
- Ova (323-339) peptide – ISQAVHAAHAEINEAGR
- OVA 257 264 peptide – SIINFEKL
- OVA 257 264 peptide SIINFEKL – KLH conjugate
- OVA 257-264 scrambled (FILKSINE)
- OVA Peptides Pool
- Ovalbumin (324-338), chicken, quail
- Ovalbumin (324-340) acetyl/amide, chicken
- ovalbumin (371-382), chicken
- P17
- PADRE peptide – AKFVAAWTLKAAA
- Palmitoyl GHK tripeptide
- PD-1 (21-35)
- PD-1 (24-38)
- PD-1 (27-41)
- Peptide antigen and epitope catalog
- Peptide Tyrosinase (Asp371) – HLA-A*0201 (YMDGTMSQV)
- Pip6a
- PLP (139-151)
- PMX 205
- PMX 53
- PRAME (100-108) HLA-A*0201
- Restricted PADRE peptide- ak(Cha)VAAWTLKAAa-Ahx-C
- RS09/Toll Like Receptor TLR4 agonist – APPHALS – CAS 1449566-36-2
- S2-16
- Se™elanotide
- SMAP-18
- SPA4 Peptide
- survivin (baculoviral IAP repeat-containing protein 5) (21-28)
- SYFPEITHI – MHC binding peptide
- TET 830 modified/T-helper epitope from tetanus toxoid – AQYIKANSKFIGITEL
- TET 830/Tetanus Toxin (830-844) peptide – QYIKANSKFIGITEL
- Tetanus Toxin P2 (830 – 844)
- TetTox-B (830-843) MHC II DRB1*07:01
- TKD (450-463)
- Tregitope 084
- Tregitope 289
- TRP-2 Peptide (180-188) – SVYDFFVWL
- Uty HY Peptide (246-254) Mouse
- V5 peptide
- Vitronectin (367-378)
- [Ala144]-PLP (139-151)
- Ion Channels and Transporters
- (Dap22)-ShK
- 8xHis-ProTx-II
- Aah-II
- Acetyl-Claudin-3
- Acetyl-Claudin-6
- Acetyl-Claudin-9
- ACT1
- ADWX-1
- Agitoxin-2
- alpha-cobratoxin
- AmmTx3
- Apamin
- APETx2
- Apolipoprotein A-I (APOA1)(86-101)
- Apolipoprotein KV domain (67 – 77)
- ATTO488-Charybdotoxin
- ATTO488-ProTx-I
- ATTO488-ProTx-II
- ATX-II
- BDS-I
- BeKm-1
- Biotin-ProTx-I
- BmP02
- C-Peptide (57-87) human
- Charybdotoxin
- Chlorotoxin
- Conantokin-G
- Crotamine
- Cy3-Crotamine
- Cy5-Huwentoxin-IV
- Cy5-ProTx-I
- Cy5-ProTx-II
- D-GsMTx4
- Dc1a
- Echistatin α1 isoform
- GAP26
- GaTx2
- GrTx1
- GsAF-1
- GsAF-2
- GsMTx4
- Guangxitoxin-1E
- Hainantoxin-III
- Hainantoxin-IV
- Hm1a
- HSA (55-66)
- HsTx1
- Huwentoxin-I
- Huwentoxin-IV
- Huwentoxin-XVI
- Iberiotoxin
- Jingzhaotoxin-34
- Jingzhaotoxin-III
- Kaliotoxin-1
- Latartoxin-1a
- Leiurotoxin-1
- Leiurotoxin-1 Dab7
- Lys-conopressin-G
- Mambalgin-1
- Margatoxin
- Maurocalcine
- Maurotoxin
- Melittin
- MitTx (MitTx-α + MitTx-β)
- Morphiceptin
- MT7 – Muscarinic Toxin 7
- NMB-1
- Obtustatin
- OD1
- Panx-1 mimetic inhibitory peptide
- Phlotoxin-1
- Phrixotoxin-2
- Phrixotoxin-3
- PNC 27
- ProTx-I
- ProTx-II
- ProTx-II-Biotin
- ProTx-III
- Psalmotoxin-1 (PcTx1)
- Purotoxin-1
- Rho-Conotoxin-TIA
- ShK – Stichodactyla toxin
- Stromatoxin-1 (ScTx1)
- Tamapin
- TAMRA-Charybdotoxin
- TAMRA-ShK
- Tertiapin Q
- Tf2 scorpion toxin
- U2-sicaritoxin-Li1a
- Waglerin-1
- Waglerin-1-FAM
- α-Conotoxin BuIA
- α-Conotoxin PIA
- α-conotoxin-GI
- α-conotoxin-GID
- α-conotoxin-IMI
- α-conotoxin-MI
- α-conotoxin-PeIA
- αC-Conotoxin-PrXA
- β-Pompilidotoxin
- µ-conotoxin KIIIA
- µ-conotoxin-CnIIIC
- µ-conotoxin-GIIIB
- μ-conotoxin-PIIIA
- µO conotoxin MrVIB
- ρ-Da1a (AdTx1)
- ω-agatoxin-IVA
- ω-Conotoxin-GVIA
- ω-Conotoxin-MVIIA
- ω-Conotoxin-MVIIC
- ω-Conotoxin-SO3
- ω-Hexatoxin-Hv1a
- ω-Tbo-IT1
- Multiple sclerosis peptides
- Biotin-Mouse MOG (35-55) peptide
- Experimental Autoimmune Encephalomyelitis KIT
- Human MOG Peptides Pool
- MBP (1-11) human: Ac-ASQKRPSQRHG CAS 106128-98-7
- MBP (84-97) – VVHFFKNIVTPRTP
- MBP (85–99) – EKPKVEAYKAAAAPA
- MOG (183-191) – FVIVPVLGP
- MOG (35-55), human peptide
- MOG (91-108) peptide – SDEGGYTCFFRDHSYQEE
- MOG (92-106) peptide – DEGGYTCFFRDHSYQ
- MOG (97-108) peptide- TCFFRDHSYQEE
- Mouse MOG (35-55) peptide
- PLP (139-151) peptide – HSLGKWLGHPDKF – [CAS 122018-58-0]
- PLP (178-191): NTWTTCQSIAFPSK mouse, rat
- Myelin Basic Protein (MBP) Peptides
- Neuroscience Peptides
- (Ala11, D-Leu15)-Orexin B human
- (Arg8) Vasopressin (AVP)
- (Arg8) Vasotocin
- (D-Pro7)-Angiotensin I/II (1-7)
- (Des-octanoyl)-Ghrelin Human
- Acein
- Acetyl-Alpha-synuclein (1-13)
- Acetylated alpha-synuclein (1-7) amide
- ACTH (1-10) Human
- ACTH (1-17) Human
- ACTH (1-24) Human
- ACTH (1-39) Human
- ACTH (11-24)
- ACTH (15-24) Cys
- ACTH (18-39) Human
- ACTH (7-38) Human
- ACTH (7-39) Cys
- Alpha-Casozepine
- alpha-MSH
- Alpha-synuclein (1-13)
- Amylin (1-37) Human
- Amyloid beta peptides
- Angiotensin (Human, 1-7)
- Angiotensin I
- Angiotensin II Antipeptide
- Angiotensin III
- Angiotensin IV (3-8)
- Apelin (65-76), human
- Apelin-17 (human, bovine)
- Apolipoprotein E fragment (133-149) – COG133 : LRVRLASHLRKLRKRLL (CAS : 514200-66-9)
- Biotin-ACTH (1-39) Human
- CCK octapeptide Cholecystokinin (26-33)
- CE dipeptide
- CMX-8933
- CRF human, rat
- Duck liver-derived peptide 2
- Duck liver-derived peptide 3
- Duck liver-derived peptide 4
- EC dipeptide
- Echinotocin neuropeptide
- FMRFamide peptide
- Galanin (1-13)
- Galanin (1-15) Porcine, Rat
- Galanin (1-17) Porcine
- Galanin (13-20) Mouse
- Galanin (2-12) acid
- Galanin (2-13)
- Galanin (2-13) acid
- Galanin (2-30) acid
- Galanin (3-13)
- Galanin Human
- Galanin Mouse, Rat
- Galantide
- Ghrelin Human
- Ghrelin Rat, Mouse
- GP dipeptide
- GRP (18-27) (human, porcine, canine)
- Hyp-Gly dipeptide
- IFNB1 (118-132) Human deimmunised
- Kinetensin
- Kisspeptin 10 human
- Kisspeptin 14 human
- KLPGF peptide
- L57
- Leptin (116-130) Mouse
- Leptin (93 – 105) Human
- LRRKtide
- MiniAp-4 peptide
- Motilin (1-16)
- Myhc-α 334-352
- Neurokinin A (Substance K)
- Neurokinin B (human, porcine)
- Neuromedin U 25
- Neuropeptide NPSF
- Neuropeptide RFRP-1 (81-92)
- Neuropeptide RFRP-2 (101-112)
- Neuropeptide RFRP-3 (124-131)
- Neuropeptide S human
- Neuropeptide S mouse
- Neuropeptide S rat
- Neuropeptide Y (3-36) Human,Rat
- Neurotensin
- Nociceptin
- NX 210
- Orexin A (monkey)
- Ovalbumin (154-159)
- Ovotransferrin (328-332)
- OXA (17-33)
- Oxidised Alpha-synuclein (1-13)
- Oxytocin (free acid)
- Pep63
- Peptide5
- Polybia-MPII
- SARS-CoV Peptide Antigen negative control
- Spexin
- Substance P
- Thyroglobulin (Tg-FSP)
- Thyroglobulin (Tg-VIF)
- VIP (1-12)
- VIP (6-28)
- [5-FAM]-Galanin (2-30)-[Cys] (Human)
- [Cys]-Galanin (1-30) Human
- [Glp6,Pro9] Substance P (6-11)/Septide
- [Pyr]-Apelin-13
- α-CGRP (mouse, rat)
- Other Categories
- 123B9
- 14-3-3 zeta/delta (28-41)
- 1D,6L-Lanthionine vasopressin
- 3x DYKDDDDK peptide
- a-Gliadin (229-246)
- AD01
- Adrenomedullin (22-52)
- AF10847
- alpha-gliadin (58-73)
- Angiotensin II
- APYTFGQGTK peptide
- Autocamtide-2
- Beclin-1
- Biotin-DAG Peptide
- BMAP-28
- BMF
- BNP-32, porcine
- Bombesin – Potent natural agonist of the mammalian receptor GRPR
- C-telopeptide
- C5aR2 agonist
- cAC 253
- Caloxin 1C2
- Cardiac Targeting Peptide CTP
- CBL (167-180) Light
- CBL (598-612) Light
- CBL-B (22-37) Light
- CBL-B (239-247) Light
- CCK Octapeptide sulfated
- Cecropin-B
- Cellulose synthase 7
- CIGB 300
- Cilengitide
- CNP (1-22), Human, Porcine
- CREB327/active transcription factor CREB-A (113-126) Biotinyl, human
- CREB327/active transcription factor CREB-A (113-126) [5-FAM] amide, Human
- CREB327/active transcription factor CREB-A (113-126), human
- Cysteine Peptide for DPRA tests
- D11-FxxLF Coactivator peptide
- DAG peptide
- Dinitrophenyl ERAP1 peptide
- DYKDDDDK peptide
- Dystrophin (2690-2700)
- Dystrophin (2765-2777)
- Dystrophin (396-405)
- Dystrophin (50-61)
- Dystrophin, DMD
- EHD1
- elf18
- Enfuvirtide (T-20)
- Farnesylated a-factor
- Fas blocking peptide
- FEFEFKFK
- Fibrinogen (377-395) Human
- Flagellin 22 (flg22)
- Formyl-MIFL-acid
- Formyl-Δ-toxin (1-26)
- FSY tripeptide
- Fz7-21
- GALA Peptide
- Ganglioside GM1-binding peptides p3
- Gastrin-1 Rat
- GG-[AMC]
- GIP (1-30) Human amide
- GPRPK pentapeptide
- GQPR tetrapeptide
- GRGDS peptide
- GS dipeptide
- GSS tripeptide
- Heart-homing peptide
- Herceptide
- HiBiT tag
- HIV-1 Rev (34-50)
- HPV16 E6 pep11**m
- HSA (549-558)
- Human Influenza Hemagglutinin (HA) Tag (YPYDVPDYA)
- humanized anti-Tac (HAT) binding peptide
- Hyp3-Bradykinin
- Ig heavy chain V-III region Light
- Ig heavy chain variable region Light
- IGRP Catalytic Subunit-related Protein (206-214)
- Insulin A Chain (A12-21)
- Insulin B (9-23)
- Integral membrane TGN38A (350-361) acetyl, mouse
- Integrin-binding cell adhesive peptide
- Intracellular Sigma Peptide
- JAG-1 (188-204)
- JAG-1, scrambled
- Jelleine 1
- Jelleine 2
- Jelleine 3
- Jelleine 4
- Kallikrein-2 inhibitor
- KLHY-[AMC]
- KR-12-a5 (6-DL)
- KRREILSRRPSYR-acid
- Lasioglossin-III
- LDVP peptide
- Leuprolide Acetate
- LLO (91 – 99)
- Locustatachykinin I
- LP2
- Lys-Glu dipeptide
- Lysine peptide for DPRA tests
- M12 muscle-homing peptide
- M2-Influenza
- MAGEA4 (230-239) Light
- MART-1 (27-35) (human)
- MART-1 Fragment
- Max-1 peptide
- Mouse-ESC-derived cardiomyocyte-targeting peptide
- Mucin 10 (153 – 165), EA2
- MyHC (614-629)
- N-L-Glutamyl-L-Lysine
- Natalizumab (LC46-58)
- Natalizumab LC46-58 KGN deimmunised
- Natalizumab LC46-58 KSN deimmunised
- nef peptide [Human immunodeficiency virus type 1] (73-82) acetyl/amide
- nef protein (75-82) [Human immunodeficiency virus 1]
- nef protein fragment (Acetyl/amide) [Human immunodeficiency virus 1]
- Neuromedin U 8
- Nictide
- Octreotide
- P007 (RXR)4XB
- P12
- P12 amide
- P1A antigen
- Palmitoyl GQPR tetrapeptide
- Palmitoyl KTTKS pentapeptide
- PEN (Mouse)
- PEN, Human
- PEN, Rat
- Pepstatin A
- Pepstatin A Biotin
- Peripheral Myelin Protein P0 (180-199)
- polyalanine peptide (pALA)
- Polybia-MPI
- Polyproline-13
- pp89 phosphoprotein fragment [Mouse cytomegalovirus 1]
- Proinsulin 90-104
- Prolactin-releasing peptide (PrRP20)
- Prostate-specific membrane antigen PSM (35-40), human
- PTD-p65-P1 Peptide
- PUMA BH3
- R5
- Relaxin 3 B1-22R amide
- RGD peptide
- RGD Peptide GRGDSPK
- Rhodopsin Epitope Tag
- S413-PV-[Cys(Npys)]
- SARS-CoV-2 Spike (411-420)
- SARS-CoV-2 Spike (976-984)
- SBCleaner – Peptide decontamination
- Semaglutide Heavy
- SG dipeptide
- SGS tripeptide
- Shepherdin (79 – 87)
- Sialokinins I
- Sifuvirtude
- Skeletal muscle-targeted peptide MSP
- SmBiT
- SMRT peptide
- SOD1 (147-153) human
- Spexin 2 (53-70) Human, Mouse, Rat
- SRC-1 (676-700)
- SSG tripeptide
- Stabilized avi tag peptide
- Steroid Receptor Coactivator-1 (SRC-1) (686-700)
- Suc-LLVY-acid
- Syntide 2
- T-9 peptide
- TAT-GSK’364A
- Teduglutide (GLP2 2G)
- Temporin SHF
- Tet-20
- Tetanus Toxin (1084-1099)
- Tetanus Toxin (1174-1189)
- Tetanus Toxin P30 (947-967)
- Thrombin Receptor Antagonist
- TNF-alpha (1-26), human
- TP10
- TPL-2tide
- Transportan
- TRAP-6 peptide
- Triptorelin acetate
- Truncated flagellin 22 (flg22)
- Tumstatin (69-88)
- Ub4ix
- UBA3 (59-72) peptide
- Urumin
- V5 epitope tag
- Vasculotide
- VGB4
- VIP (guinea pig)
- Visperas1pY
- Visperas2pY
- Xenin
- YSA amide
- [5-FAM]-DAG peptide
- [Azhx]-ANP (Human)
- [Cys] citrullinated alpha enolase
- [Cys]-Exendin 4
- [Nle12] a-factor
- [Tyr]-CNP22, Human
- εV1-2
- Protein-Protein Interactions
- Stable Isotope Labeled (SIL) peptides catalog
- ADALQAGASQFETSAAK* – SIL Infliximab signature peptide
- APYTFGQGTK* – SIL Adalimumab internal standard
- ASGYTFTSYNMHWVK* – SIL Rituximab signature peptide quantifier
- ASQSIGTNIHWYQQR* – SIL Cetuximab signature peptide qualifier
- DYAMTWVR* – SIL Dupilumab signature peptide qualifier
- GLEWIGAIYPGNGDTSYNQK* – SIL Rituximab signature peptide qualifier
- Heavy Angiotensin II
- Heavy calcitonin peptide
- LEWIGEIDPSESNTNYNQK* – SIL Vedolozumab signature peptide
- LSITIRPR* – SIL Dupilumab signature peptide quantifier
- NYLAWYQQKPGK* – SIL Adalimumab signature peptide quantifier
- QAPGQGLEWMGDINTR* – SIL Emicizumab signature peptide qualifier
- SGGSIYNEEFQDR* – SIL Emicizumab signature peptide quantifier
- SINSATHYAESVK* – SIL Infliximab signature peptide quantifier
- SLEWIGAIDPYYGGTSYNQK* – SIL Dinutuximab signature peptide qualifier
- SSSTAYMHLK* – SIL Dinutuximab signature peptide quantifier
- YASESISGIPSR* – SIL Cetuximab signature peptide quantifier
- YASESMSGIPSR* – SIL Infliximab signature peptide qualifier
- TAT Conjugated Peptides
- Cys-TAT(48-60)
- dfTAT
- fTAT
- gp91 ds-TAT
- SMAC/DIABLO -TAT (48-60)-[Lys]
- TAT (48-57)
- TAT (48-59) amide
- TAT (48-60) amide
- TAT – GluR23Y
- TAT 2-4
- TAT protein (28-35) [Simian immunodeficiency virus]
- TAT-AKAP79 (326-336) amide
- TAT-AKAP79 (326-336) scrambled
- TAT-AKAP79 (326-336) scrambled amide
- TAT-Beclin 1
- TAT-Beclin Scrambled
- TAT-CHN9 (C-ter)
- TAT-CN21
- TAT-NR2B (C-ter)
- TAT-Pro ADAM10 (709-729)
- TAT-TRPV1 (736-745)
