Bioconjugation of peptides, proteins, antibodies, oligos
Several valuable bio-conjugation reactions are routinely employed in laboratories with benefits and inconveniences.
The bioconjugation strategy must be selected according to reactive functions present in the biologic compound. Common reactive functions used are free amines, free carboxyls, free thiols.
Amine / Carboxyl conjugation
Free amines and carboxyl are present in some extremities or respectivelly in lysine’s and Asp/Glu’s side chains. They can be conjugated to other compounds containing reactive functions or following an activation (NHS ester, isocyanate, EDC…).
Thiol / maleimide conjugation
Free cysteines can react with maleimide-activated compounds. The reaction is simple and very efficient. SB-PEPTIDE is frequently using it when preparing KLH-peptides and BSA-peptides.
These bioconjugation strategies are very efficient, selective and cost effective. Nevertheless they can not always be employed. As simple example, if we have an objective to conjugate one fluorescent at the N-ter extremity of a peptide but this peptide already contains lysines in its sequence, an amine conjugation can not be considered. Alternatives are the introduction of a biotin or a maleimide or a clickable function.
Biotin / Streptavidin conjugation
Bioconjugation using biotin will react specifically with a streptavidin. This reaction is widely used especially in the field of protein bioconjugation. In the field of peptide, peptide biotinylation is routinely employed for ELISA or pull-down applications but less recommended for conjugation of fluorescent dyes for instance, as streptavidin size will too much impact the peptide movments or binding properties.
Click chemistry conjugation
In some cases, click-chemistry is a valuable alternative to avoid all other inconveniences. It is highly selective, yields are good and the crosslinker is small. It remains more difficult to manage as the biologic compound musts contain a clickable function which is nevertheless quite easy in peptide and oligo synthesis.
Developped in the early 2000s, click-chemistry is a very specific chemical conjugation technic. The main reaction consists in associating an alkyne group to an azide group thanks to a copper catalysed cycloaddition reaction. This reaction is highly selective, meaning that no other chemical functions can react with azide and alkyne groups. Click-chemistry is widely used in bio-conjugations of chemicals, peptides and oligonucleotides as they are usually chemically synthesized so introduction of alkynes and azides are simple. Proteins (including enzymes and antibodies) can now also be azide / alkyne functionalized thanks to the incorporation during the biosynthesis of modified amino acids using adapted strains.
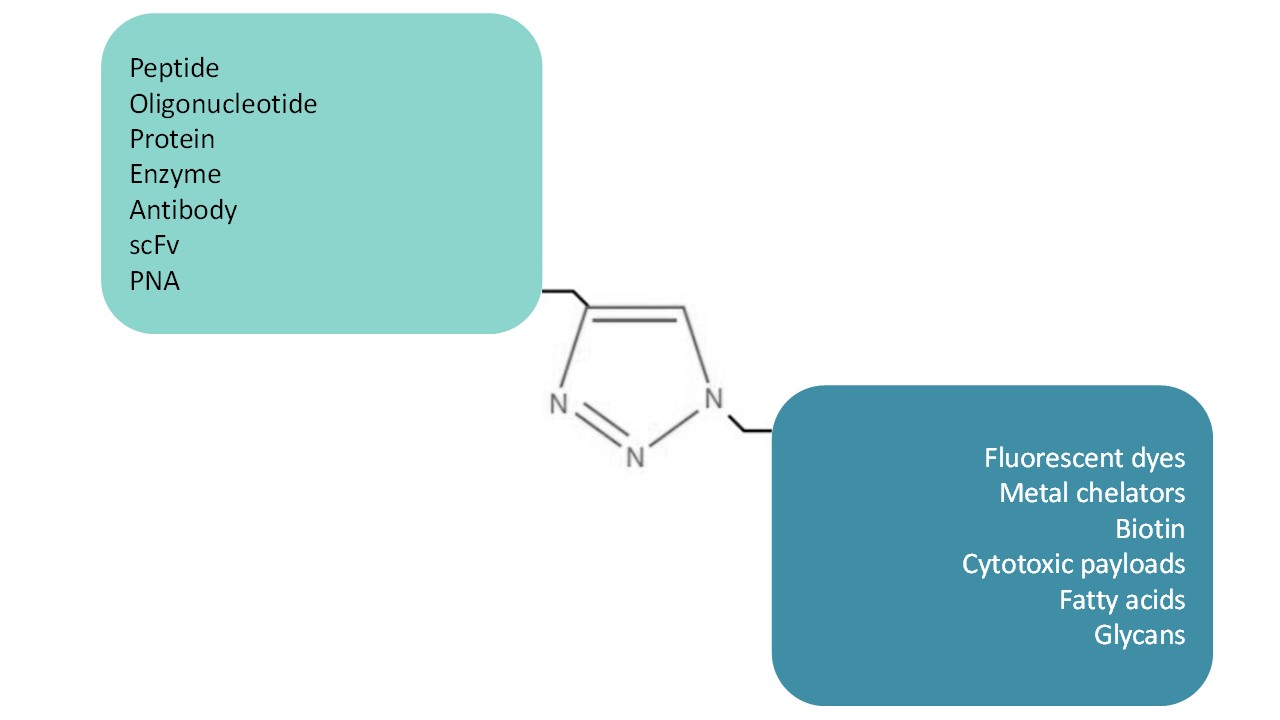
SB-PEPTIDE Bio-conjugation service
SB-PEPTIDE is performing in routine all kind of bio-conjugations for many years on peptides as well as proteins, oligonucleotides, PNAs, single domain antibodies (sdAb), scFv and antibodies. The company is fully equiped with purification (RP-HPLC, SEC-HPLC) and analytical systems (LCMS, LCMSMS).
SB-PEPTIDE can conjugate a wide range of compounds including fluorescent dyes, biotin and desthiobiotin, metal chelators (DOTA, NOTA, Ruthenium cages), fatty acids, cytotoxic payloads.
It can also conjugate several biologics together to form homodimers or heterodimers of peptides, peptide-oligonucleotides conjugates…
SB-PEPTIDE can help clients to define the best conjugation strategy so please get in touch
The Nobel Prize in Chemistry 2022
Chemists have long been driven by the desire to build increasingly complicated molecules. In pharmaceutical research, this has often involved artificially recreating natural molecules with medicinal properties. This has led to many admirable molecular constructions, but these are generally time consuming and very expensive to produce.
“This year’s Prize in Chemistry deals with not overcomplicating matters, instead working with what is easy and simple. Functional molecules can be built even by taking a straightforward route,” says Johan Åqvist, Chair of the Nobel Committee for Chemistry.
Barry Sharpless – who is now being awarded his second Nobel Prize in Chemistry – started the ball rolling. Around the year 2000, he coined the concept of click chemistry, which is a form of simple and reliable chemistry, where reactions occur quickly and unwanted by-products are avoided.
Shortly afterwards, Morten Meldal and Barry Sharpless – independently of each other – presented what is now the crown jewel of click chemistry: the copper catalysed azide-alkyne cycloaddition. This is an elegant and efficient chemical reaction that is now in widespread use. Among many other uses, it is utilised in the development of pharmaceuticals, for mapping DNA and creating materials that are more fit for purpose.
Carolyn Bertozzi took click chemistry to a new level. To map important but elusive biomolecules on the surface of cells – glycans – she developed click reactions that work inside living organisms. Her bioorthogonal reactions take place without disrupting the normal chemistry of the cell.
These reactions are now used globally to explore cells and track biological processes. Using bioorthogonal reactions, researchers have improved the targeting of cancer pharmaceuticals, which are now being tested in clinical trials.
Click chemistry and bioorthogonal reactions have taken chemistry into the era of functionalism. This is bringing the greatest benefit to humankind.
Click Chemistry: Diverse Chemical Function from a Few Good Reactions
Examination of nature’s favorite molecules reveals a striking preference for making carbon-heteroatom bonds over carbon-carbon bonds-surely no surprise given that carbon dioxide is nature’s starting material and that most reactions are performed in water. Nucleic acids, proteins, and polysaccharides are condensation polymers of small subunits stitched together by carbon-heteroatom bonds. Even the 35 or so building blocks from which these crucial molecules are made each contain, at most, six contiguous C-C bonds, except for the three aromatic amino acids. Taking our cue from nature’s approach, we address here the development of a set of powerful, highly reliable, and selective reactions for the rapid synthesis of useful new compounds and combinatorial libraries through heteroatom links (C-X-C), an approach we call « click chemistry ». Click chemistry is at once defined, enabled, and constrained by a handful of nearly perfect « spring-loaded » reactions. The stringent criteria for a process to earn click chemistry status are described along with examples of the molecular frameworks that are easily made using this spartan, but powerful, synthetic strategy.
Click Chemistry: Diverse Chemical Function from a Few Good Reactions, 2001 Jun 1;40(11):2004-2021.
doi: 10.1002/1521-3773(20010601)40:11
